
The BD OnclarityTM HPV Assay provides extended high-risk HPV genotyping results beyond 16 and 18, to support risk stratification and enhanced patient management

The BD OnclarityTM HPV Assay provides extended high-risk HPV genotyping results beyond 16 and 18, to support risk stratification and enhanced patient management
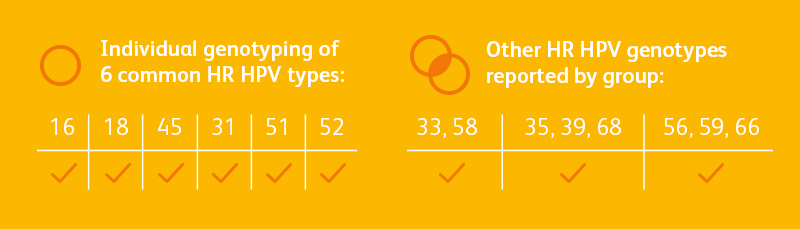
The BD OnclarityTM HPV Assay provides extended genotyping, offering the flexibility you need to adapt to changing clinical landscape and screening guidelines, including self-collection



1. Stoler MH et al. Gynecol Oncol. 2019;153(1):26–33. 2. Bonde J et al. Int J Cancer. 2019; doi:10.1002/ijc.32291. 3. Elfgren K et al. Am J Obstet Gynecol. 2017;216(3):264.e1–264.e7. 4. Radley D et al. Hum Vaccin Immunother. 2016;12(3):768–772. 5. Bottari F et al. J Low Genit Tract Dis. 2019;23(1):39–42. 6. World Health Organization, Department of Reproductive Health and Research. Cervical cancer, human papillomavirus (HPV), and HPV vaccines—key points for policy-makers and health professionals. 2007. 7. Oliver SE et al. J Infect Dis. 2017;216(5):594–603. 8. Drolet M et al. Lancet Infect Dis. 2015;15(5):565–580. 9. Garland SM et al. Clin Infect Dis. 2016;63(4):519–527. 10. Schiffman M et al. Gynecol Oncol. 2015;138(3):573–578. 11. Schiffman M et al. Int J Cancer. 2016;139(11):2606–2615. 12. BD Onclarity™ HPV Assay EU Package Insert (8089899). 13. Tjalma WAA et al. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):45–46.